ARTICLE
AI in clinical trials presents a data-driven model
Bloomberg Intelligence
This analysis is by Bloomberg Intelligence Industry Analyst Andrew Galler and Senior Associate Analyst Jack Maltby. It appeared first on the Bloomberg Terminal.
Clinical trials represent a significant portion of the drug development cycle, accounting for over half of the total time and cost, but the overall process has remained little changed in recent decades. We think the integration of artificial intelligence should allow drug developers to take a more data-driven, quantitative approach to clinical trial conduct that can drive efficiencies without negatively affecting a program’s probability of success.
Expanding eligibility without sacrificing efficacy
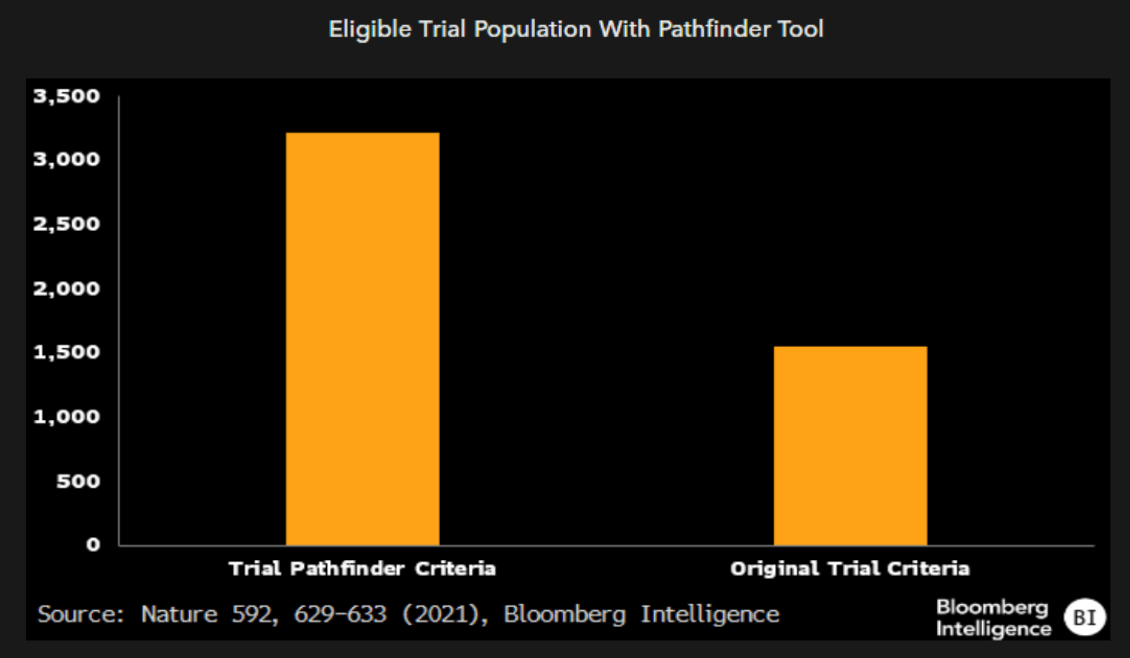
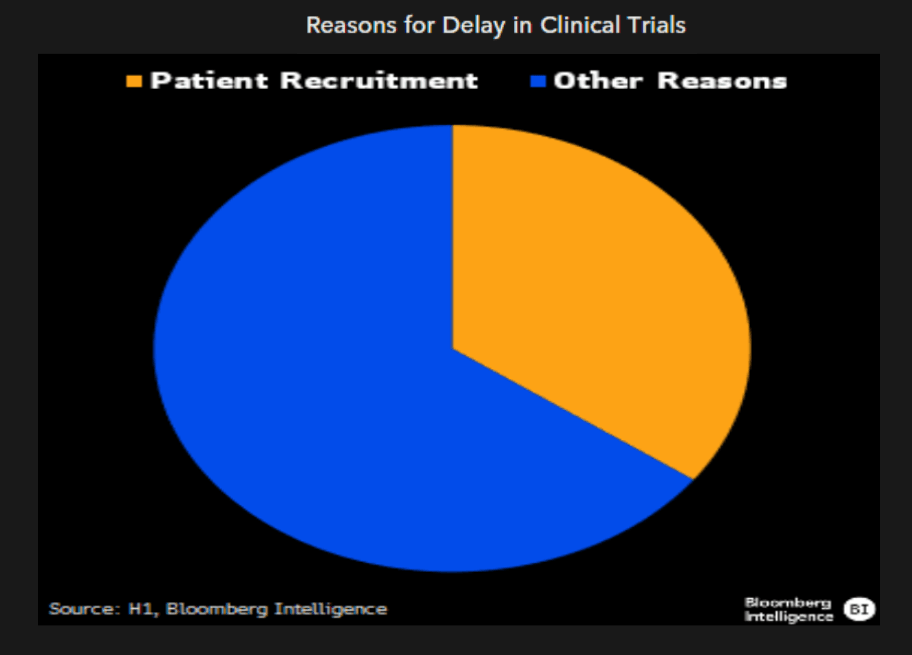
Aside from disease-specific criteria, most clinical trials have sets of inclusion-exclusion criteria that’s largely driven by heuristics. These include lab measures, such as liver enzymes and white blood cell counts, that are rarely expected to significantly impact efficacy but rather exclude patients with separate, underlying conditions. We think by training AI on prior clinical trial datasets, drug developers can take a data-driven approach to enrollment criteria without sacrificing efficacy, potentially increasing the speed of enrollment, which is the longest part of most trials.
In one such data-driven approach for non-small cell lung cancer trials, the model was able to double the eligible trial population with no estimated impact on efficacy. Indeed, some models even boosted predicted efficacy.
Identifying potential super-responders with AI models
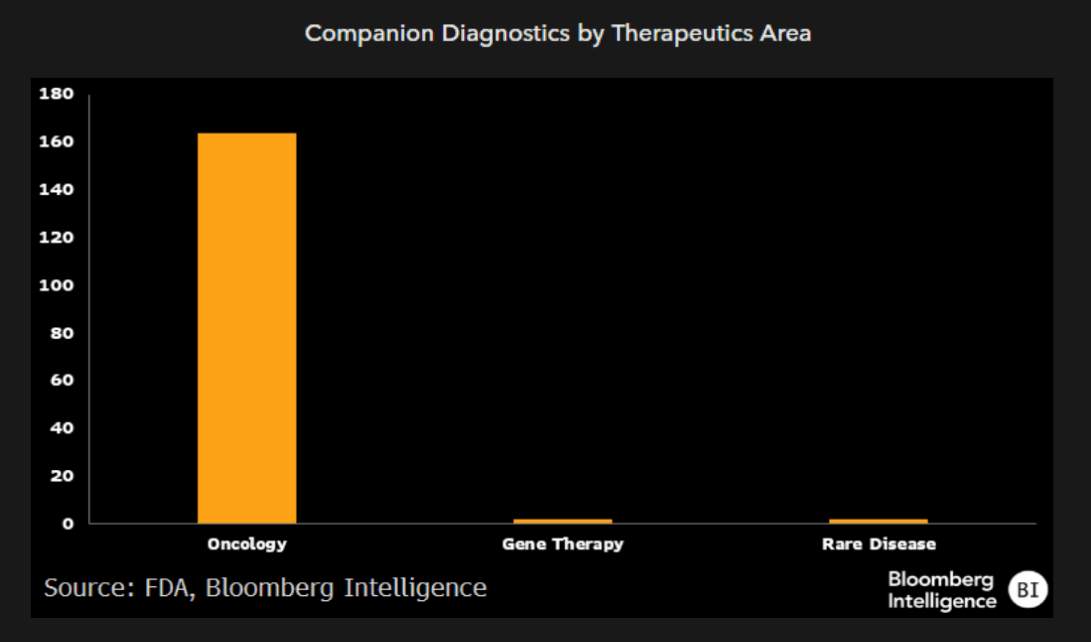
Particularly in crowded therapeutic areas, such as immunology or oncology, biopharmaceutical companies have been working on ways to further segment markets by identifying patients most likely to respond to different mechanisms. AI has the potential to comb through large amounts of genomics and biomarker data and not only identify predictive factors for efficacy, but also set thresholds to optimize segmentation of the patient population and ensure drug developers target feasible market opportunities. AI may also enable use of new methods by allowing for real-time quantitative assay work that may not have been feasible for physicians to run by themselves.
We see a secondary benefit to diagnostics companies as many of these strategies will also require commercially available companion diagnostics.
In-focus: AstraZeneca’s AI-enabled TROP2 biomarker
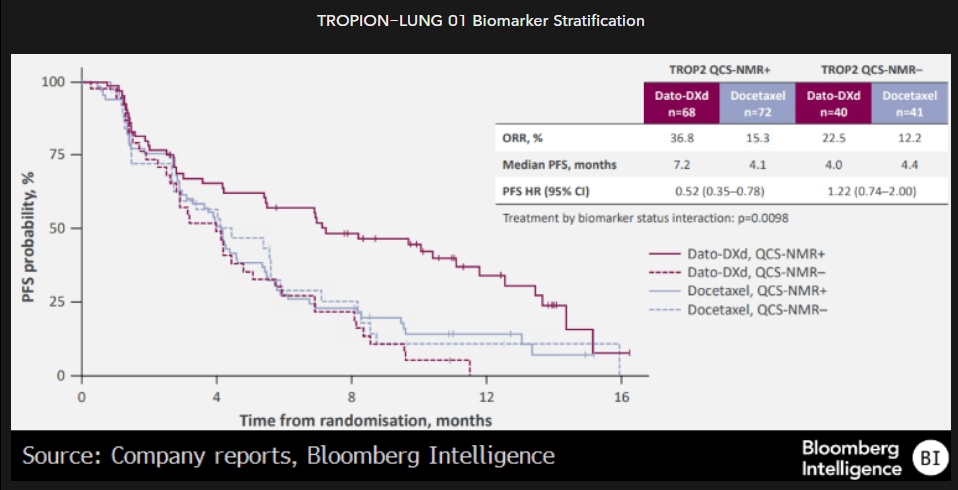
AstraZeneca’s datopotamab deruxtecan is a TROP2-targeting antibody-drug conjugate (ADC) in development for multiple solid tumors. An issue in the development of TROP2-targeting ADCs has been a lack of correlation between TROP2 expression on standard IHC measures and efficacy, which runs contrary to standard biomarker experience. AstraZeneca took a different tack, looking at the ratio of membrane-bound TROP2 to total TROP2 (membrane and cytoplasm) and using algorithmic methods, determined a threshold value of 0.56 in at least 75%, which was predictive of improved efficacy.
In an analysis of TROPION-LUNG 01, the TROP2 QCS-NMR was significantly associated with improved response, based on response rates and progression-free survival.
Facilitating use of novel endpoints with AI/ML
In the standard clinical trial paradigm, functional endpoints are typically assessed at clinical visits as a discrete measure. This introduces confounding factors, such as patient effort, that may inflate the placebo response or distort the picture of a candidate’s efficacy relative to continuously measured endpoints.
AI integration with wearable monitors or sensors makes use of massive continuous datasets feasible and is likely to provide a better indicator of clinical benefit in realworld practice. Proponents of digital biomarkers have also indicated that they have the potential to allow developers to run smaller, faster clinical trials.
AI-enabled biomarkers could also supplant patient-assessed measures, which are highly susceptible to bias due to factors like functional unblinding or placebo effects.
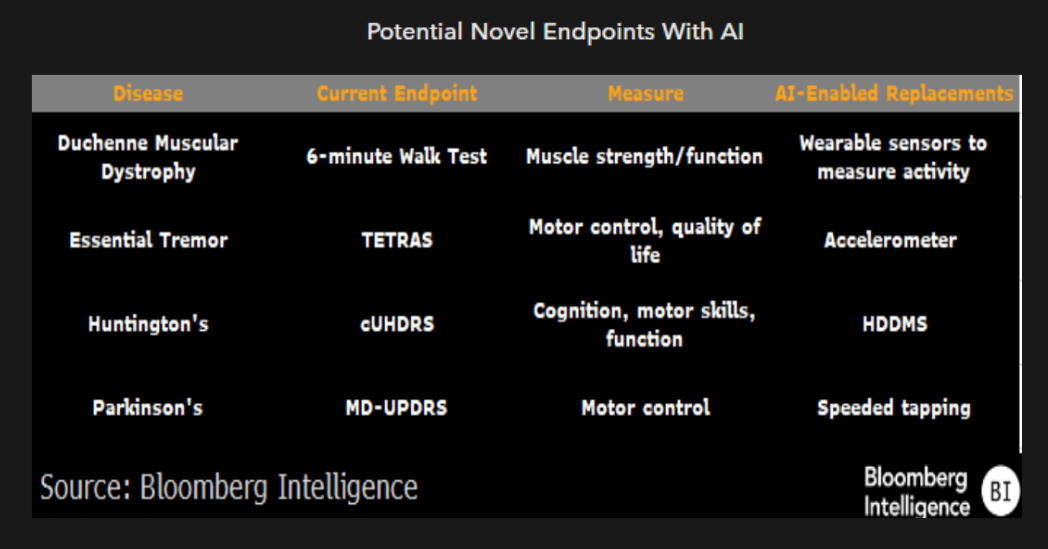
Additional optimizations enabled by AI/ML
Beyond tailoring trial designs to optimize probability of success, we see a number of additional avenues for trained models to accelerate the clinical-trial process. These include the use of deidentified medical records to identify optimal clinical-trial sites, automated data collection and analysis for outcomes like tumor shrinkage or spirometry, and improved patient adherence and adverse event reporting. AI has significant potential to improve data quality while cutting trial timelines and potentially reducing the number of patients needed for a trial.
Though these improvements individually are relatively incremental, particularly for large biopharma companies, we think taken together, they can move the needle on timelines and costs associated with clinical trials.
Digital twins for clinical trials
Among the ways AI may be integrated into clinical-trial conduct, use of digital twins is one of the more “out of the box ideas.” Digital twins — virtual replicas of objects used in simulations — have been suggested to contextualize open-label, single-arm trial data by comparing treatment efficacy to simulated data for how patients would perform if they had not received therapy. Taken further, some AI companies have indicated their plans to progress digital twins as the control arms in clinical trials, though we don’t see regulators being amenable in the near term.
Digital twin integration is attractive as a way to better contextualize trial data, particularly in cases where a matched natural history cohort may have been necessary, while controlling for between-group differences.
The data included in these materials are for illustrative purposes only. The BLOOMBERG TERMINAL service and Bloomberg data products (the “Services”) are owned and distributed by Bloomberg Finance L.P. (“BFLP”) except (i) in Argentina, Australia and certain jurisdictions in the Pacific Islands, Bermuda, China, India, Japan, Korea and New Zealand, where Bloomberg L.P. and its subsidiaries (“BLP”) distribute these products, and (ii) in Singapore and the jurisdictions serviced by Bloomberg’s Singapore office, where a subsidiary of BFLP distributes these products. BLP provides BFLP and its subsidiaries with global marketing and operational support and service. Certain features, functions, products and services are available only to sophisticated investors and only where permitted. BFLP, BLP and their affiliates do not guarantee the accuracy of prices or other information in the Services. Nothing in the Services shall constitute or be construed as an offering of financial instruments by BFLP, BLP or their affiliates, or as investment advice or recommendations by BFLP, BLP or their affiliates of an investment strategy or whether or not to “buy”, “sell” or “hold” an investment. Information available via the Services should not be considered as information sufficient upon which to base an investment decision. The following are trademarks and service marks of BFLP, a Delaware limited partnership, or its subsidiaries: BLOOMBERG, BLOOMBERG ANYWHERE, BLOOMBERG MARKETS, BLOOMBERG NEWS, BLOOMBERG PROFESSIONAL, BLOOMBERG TERMINAL and BLOOMBERG.COM. Absence of any trademark or service mark from this list does not waive Bloomberg’s intellectual property rights in that name, mark or logo. All rights reserved. © 2024 Bloomberg.